Application of dipole moment use for calculation of ionic character of covalent bonds, bond angle, electric polarization, and polarity of bond in the molecule. For example, the homonuclear non-polar diatomic molecules like molecular hydrogen, oxygen, and nitrogen define zero dipole moment but for CO, water, methane, ammonia, we use group moment to calculate the net dipole moment and polarity of the molecule in chemistry.
- When a chemical bond is formed between two identical atoms, the bonding electron equally sharing by two atoms. Therefore, the centers of gravity of the two-electron and nuclei therefore coincide.
- But for two dissimilar atoms, two electrons are not symmetrically disposed. Because each atom has a different attraction force for electron.

When chlorine and bromine combine to form covalent HBr, the electron forming the covalent bond displaced towards the bromine atom without any separation of the nucleus of an atom.
Ionic character of molecules
Application of dipole moment data uses for the determination of the ionic or covalent character of bond for heteronuclear diatomic molecules.
Let us consider compound HCl having the observed dipole moment = μobs and the bond length l cm.
If we consider HCl is a purely ionic compound the charge on H and Cl = 4.8 × 10-10 esu. Thus the dipole moment for this ionic compound
= (4.8 × 10-10) ℓ esu cm
The original dipole moment differs from the calculation and this data used to calculate the percentage of ionic character of covalent molecules.

where l = bond length of the polar molecule.
Electric Polarization and Dipole Moment
Application of electric polarization used for the calculation of the bond polarity and radius of molecules in chemistry. Thus the induced electric polarization formula for the dipole moment
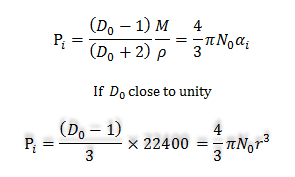
At NTP, M/ρ = molar volume = 22400cc/mole and for spherical molecule, αi= r3.
r3 = (22400/4πN0)(D0 -1)
= 2.94 × 10-21 (D0 -1)
When the known value of capacitance, we can easily determine the radius and polarity by electric polarization formula in chemistry.
Calculation of Dipole Moment
The dipole moment application uses to define the structure, bond angle, and polarity of different molecules in chemistry. For mono-atomic noble gases are non-polar because the charge of the constituent atom is distributed symmetrically.
Polarity of the Diatomic Molecules
Polarity homonuclear diatomic molecules like nitrogen, oxygen, and chlorine have zero because of the symmetrical charge distributions.
Hydrogen bromide and hydrogen iodide have non zero dipole moment indicates the unsymmetrical charge distribution between two bonding atoms.
Due to the difference in electronegativity of the constituent atoms in heteronuclear diatomic molecules always polar. Thus the electron pair is not equally shared and shifted to the more electronegative atom.
| HCl | 1.03 Debye |
| HBr | 0.79 Debye |
| HI | 0.38 Debye |
| HF | 2.00 Debye |
Polarity of Carbon Monoxide
The electronegativity difference between carbon and oxygen in CO is very large but the polarity of carbon monoxide very low.
This suggested that the electron chargedensity in the oxygen atom somehow back-donated to the carbon atom. Hence CO formed a coordinate covalent bond directing towards carbon atom to show polarity.
How to Calculate the Dipole Moment of a Molecule?
Carbon dioxide, barium chloride, stannous chloride have zero dipole moment indicating that the molecules have a symmetrical electric charge distribution between the bond.
In carbon dioxide, one carbon-oxygen cancels the bond moment of the other carbon-oxygen bond. But the bond moment associated with the bond arising from the difference of electronegativity. Thus the vectorial addition of the bond moments uses to calculate the dipole moment of the molecule.
where m1 and m2 are the bond moments.
Bond moments calculation help finding bond angle of carbon dioxide molecule, where µ = 0 and m1 = m2 = m.
or, θ = 180°
For example, water and hydrogen sulfide molecule non-polar because they have non-linear structures. The bond angle can be calculated from the polarity of the molecules.
Dipole Moment of the Water Molecule
Due to the non-linear structure of the water molecule, we can calculate the net electric dipole moment from the bond moment of water, which ≠ 0.
If the dipole moment of the water molecule
μ = 1.84 D and bond moment = 1.60 D.
∴ μ2 = 2 m2 (1+ cosθ )
or, (1.84)2 = 2 (1.60)2 (1+ cosθ )
∴ θ = 105°
Therefore the contribution of non-bonding electron toward the total dipole moment included within the bond moment of water.
Polarity of boron trifluoride
Boron trichloride, boron trifluoride are the tetratomic compound having dipole moment zero, indicating that they have regular planar structure.

Halogen atoms are on a plane at the corner of the equilateral triangle and boron atom at the intersection of the molecules. Thus the μ of the above molecules is zero.
Polarity of Ammonia and Phosphine
Other types of the molecule such as ammonia and phosphine are polar, where μ≠0 indicated that the molecule has a pyramidal structure. Hence three hydrogen atoms on a plane and nitrogen atom at the apex of the pyramid in ammonia and phosphine.
But NF3 shows a very small bond moment although there is a great difference of electronegativity between nitrogen and fluorine atoms and similar structure of NH3.
Thus this low value of µ in NF3 explained by the fact that the resultant bond moment of the three nitrogen – fluorine bonds are acting in the opposite direction to that of the lone pair placed at the nitrogen-atom. But in NH3, the resultant bond moment is acting in the same direction as that of the lone pair electrons.
Dipole Moment Penta atomic Molecule
Methane, carbon tetrachloride, platinum chloride are examples of Penta-atomic molecules having zero dipole moment.
This suggests that the molecules either regular tetrahedral or square planer structure. But polar molecules of this type have pyramidal structures.
Dipole Moment of Methane
For calculating bond polarity, let us discuss the structure of methane that has regular tetrahedral structure and the angle of each H-C-H = 109°28ˊ.
Therefore the application group moment of C-H bond provides the arrangement of the bonds. Where the difference of the electronegativity of the constituent atoms forming the bonds in the group.
But it can be shown that the group moment of methyl group identical to the bond moment of a carbon hydrogen bond. Therefore, two bond moments cancel each other and use for calculation of dipole moment of methane molecules.
∴ mCH3 = 3 mCH × Cos(180° -109°28՛)
= 3 mCH Cos 70°32՛
= 3 mCH × (1/3)
So, mCH3 = mCH
= mCH (1 + 3 Cos 109°28՛)
= 0
Dipole Moment of CH3Cl and CHCl3
μ2 = m12 + m22 + 2 m1m2 Cosθ
But here θ = 0° hence Cosθ = 1
Thus μ2 = m12 + m22 + 2 m1m2
= (m1 + m2)2
∴ μ = (m1 + m2)
= (mCH3 + mCl)
= (1.5 D + 0.4 D)
Thus μCH3Cl = 1.9 D
But for CHCl3
= (mCCl3 + mCH)
= (1.5 D + 0.4 D)
Hence μCHCl3 = 1.9 D
A similar calculation is done for polarity hydrocarbon like C2H4, C3H7, C4H9, etc in organic chemistry. Thus the group moment of such molecules = μCH.





























No comments:
Post a Comment